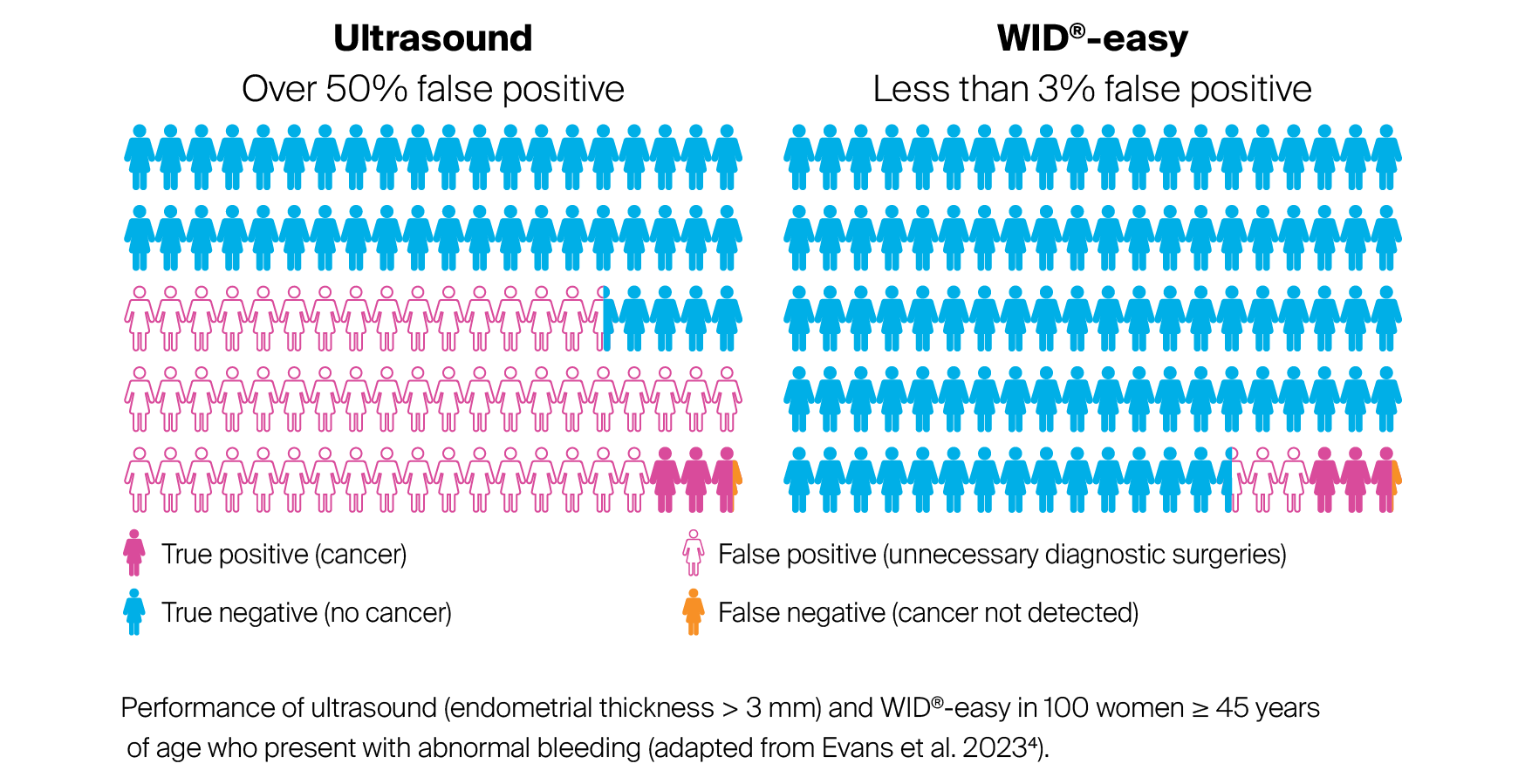Epigenetic test to detect endometrial cancer
Unusual bleeding is the main symptom in 90% of women with endometrial cancer. However, endometrial cancer can be determined as the cause in only 3% of peri- and postmenopausal cases of bleeding1. The current S3 Guideline2 recommends subjective procedures such as clinical examinations, cytology, and transvaginal ultrasounds for the differential diagnosis of endometrial cancers with unusual bleeding3. Transvaginal ultrasound has a very low positive predictive value of 4.9%4. As a result, most women who undergo a surgical diagnostic procedure do not actually have endometrial cancer.
The WID®-easy test offers the possibility of improving this situation and very quickly arriving at a diagnosis. The test demonstrates sensitivity comparable to that of transvaginal ultrasound, but reduces the rate of false positive results by 94%.
High false-positive rate in previous diagnostic testing
The current S3 Guideline2 recommends subjective and experience-based procedures such as clinical examinations, cytology, and transvaginal ultrasounds to rule out or diagnose endometrial cancer when there is abnormal bleeding.
The sensitivity of cytology in detecting endometrial cancer is inadequate, at 45% in symptomatic women8 and only 26% in asymptomatic women who have had a Pap test up to three years before diagnosis3.
Transvaginal ultrasound measures the endometrial thickness, with a thickness of more than 3 mm being considered pathological in postmenopausal women. The detection rates vary between white women (89.5%) and black women (51.1%)9. The specificity of this measurement varies between 25.7%9 and 42.1%10, and the positive predictive value is only 4.9% to 7.3%4, 10. Additionally, the ability of ultrasound to detect serous endometrial cancers is particularly poor, as around 25% of these aggressive cancers are not accompanied by increased endometrial thickness5.
This means that many women who undergo a surgical diagnostic examination do not have cancer. In this light, the development of a test for improved diagnosis of endometrial cancer is of great significance. This test should be easy to perform, enable objective and fast, automated analyses, have sensitivity comparable to that of ultrasound, and at the same time offer significantly increased specificity.
Significantly improved specificity thanks to WID®-easy
Thanks to this new test procedure, the false-positive results that frequently occur with ultrasound can be significantly reduced. The study results for WID®-easy are convincing, meaning that patients can be diagnosed more effectively and treated earlier, and unnecessary interventions and healthcare costs can be reduced.
The study, conducted with over 700 cervical swabs, showed that the epigenetic test offered a sensitivity similar to ultrasound, but had a significantly higher specificity compared to qualitative ultrasound assessment. The detection of endometrial cancers in the cohorts studied here did not seem to depend on histology, grade, stage, age, ethnicity, or menopause status. Early stages of endometrial cancer and even non-endometrioid cancers can be reliably detected thanks to WID®-easy. Above all, serous endometrial cancers can be identified with a sensitivity of 97%11. Furthermore, the sensitivity of the WID®-easy test is significantly superior to cytology, and endocervical cancers can also be more easily detected with it12.
In another study, the test was validated in a cohort of women ≥ 45 years of age with abnormal bleeding. Of the 474 symptomatic women, 400 agreed to participate in the study. Transvaginal ultrasound alone was conclusive in 62% of patients, while 38% of patients required additional imaging tests. Histological work-up was recommended in 47% of study participants, with 3% being diagnosed with cancers. WID®-easy demonstrated a sensitivity of 91% and a high specificity of 97%, while ultrasound had a sensitivity of 91% and a lower specificity of 46%. Interestingly, two cancers were not detected by hysteroscopy and curettage (only later by hysterectomy or biopsy of a liver metastasis), while the WID-qEC test was positive4.
Comparison of ultrasound and WID®-easy
In summary, considering the current guidelines and with optimal ultrasound diagnostic testing, for 100 women in the age group ≥ 45 years who present due to abnormal bleeding, around 56 women must undergo surgical diagnostic work-up in order to identify 3 cancers. In contrast, when using WID®-easy, only around 6 women with a positive test result need to undergo diagnostic surgery to identify the 3 cancers. As a result, WID®-easy reduces the rate of false-positive results by 94%.